Definition of Surface Energy
Surface energy is a fundamental concept in the field of microfluidics as it plays a significant role in the behavior of fluids at the microscale because the high surface area-to-volume ratio in microfluidic channels means surface effects can dominate over bulk properties. This impacts factors such as flow rate, droplet formation, and the interaction of the fluid with the channel walls. Understanding this concept of surface energy is crucial for designing and optimizing microfluidic devices for a wide range of applications, from medical diagnostics to chemical synthesis.
Understanding Surface Energy
Surface energy measures the excess energy at a material’s surface compared to its bulk. Molecules at the surface of a material are in a higher energy state and experience therefore net inward forces that tend to minimize the surface area. These forces are balanced by the resistance of the material to deformation leading to a state of tension similar to a stretched elastic band.
This results in surface tension in liquids, driving phenomena like droplet formation where the liquid assumes a spherical shape to minimize surface area.
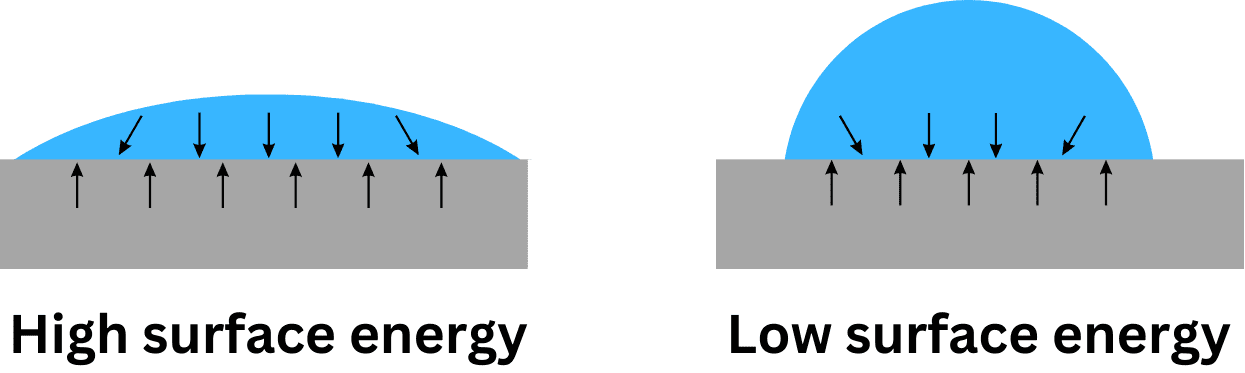
Measurement of Surface Energy
Surface energy can be measured using various methods, depending on the nature of the material and the specific application.
One common method is the contact angle measurement, which involves placing a droplet of a known liquid on the surface of the material and measuring the angle at which the liquid meets the surface. A smaller contact angle indicates a higher surface energy. This method can be used to characterize the wettability of microscale channels, which influences the flow behavior of the fluid.
Another method for measuring the surface energy is the capillary rise method, which involves immersing a narrow tube in a liquid and observing how high the liquid rises in the tube due to surface tension balancing gravity. By measuring the height of the liquid column and knowing the density and surface tension of the liquid, the surface energy of the tube material can be calculated.
Factors Influencing Surface Energy
Several factors affect surface energy, including the nature of the material, the surface roughness, and the presence of any surface treatments or coatings. For example, hydrophobic materials, such as Teflon, have a low surface energy and resist wetting, while hydrophilic materials, like glass, have a high surface energy and are easily wetted by water.
Surface roughness generally increases effective surface area and surface energy, with rough surfaces generally having a higher surface energy than smooth surfaces, though the relationship can be complex and depending on the size and shape of the roughness features.
Role of Surface Energy in Microfluidics
In microfluidic systems, surface energy impacts the fluid behavior. One of its key effects is the capillary action, which is the ability of a liquid to flow in narrow spaces against gravity. This results from the balance between adhesive forces, attracting the liquid to the channel walls, and cohesive forces, holding the liquid molecules together.
Surface Energy and Flow Behavior
High surface energy channels promote wettability, resulting in uniform flow profiles ideal for mixing applications. Conversely, low surface energy channels can lead to plug flow, where central fluid moves faster than near the walls, useful in separation processes.
Surface energy can also influence the flow rate of the fluid in microfluidic systems with lower surface energy generally leading to faster flow due to reduced friction between the fluid and the channel walls. However, this relationship depends on a number of factors, including the viscosity of the fluid, the size and shape of the channel, and the pressure gradient driving the flow.
Surface Energy and Droplet Formation
Surface energy also affects the formation of droplets. The size and shape of the droplets are determined by the balance between the surface tension of the liquid, which tends to minimize the surface area of the droplet, and the shear forces applied by the flow, which tend to deform the droplet. Therefore, by controlling the surface energy of the channel walls, it is possible to control the droplet formation process.
Applications of Surface Energy in Microfluidics
Understanding and controlling surface energy is crucial for a wide range of applications in microfluidics.
Medical Diagnostics
One of the most important applications is in the design of microfluidic devices for medical diagnostics. These devices often involve the manipulation of complex biological fluids, like blood or saliva, where surface energy plays a crucial role in controlling fluid flow, component separation, and reagent interactions. For example, in a microfluidic device for blood analysis, the surface energy of the device can influence the separation of the blood cells from the plasma, the mixing of the blood with the reagents, and the detection of the analytes.
By optimizing surface energy, these devices can enhance the sensitivity and specificity of diagnostic tests, enabling more accurate measurements of biological markers. Moreover, varying surface energy across different materials allows for the integration of multiple functions—such as sample preparation, reaction, and detection—into a single multifunctional device.
Chemical Synthesis
In chemical synthesis, microfluidic devices offer precise control over reaction conditions, where surface energy significantly influences reactant mixing, heat transfer, and product separation, all of which can affect the yield and purity of the synthesized chemicals. For example, in a microfluidic device for nanoparticle synthesis, surface energy can affect particle nucleation and growth, their dispersion within the fluid, and their subsequent separation from the fluid.
By managing surface energy, it is possible to control the synthesis of nanoparticles and other complex chemicals, affecting their size, shape, and uniformity. This control enables the production of high-quality materials with specific properties, useful in various applications such as drug delivery and electronics.
Conclusion
Surface energy is a key factor in microfluidics, influencing phenomena like capillary action and droplet formation. Mastery of surface energy is vital for optimizing microfluidic devices across applications, from medical diagnostics to chemical synthesis. As research advances, techniques for controlling surface energy are expected to become more precise, further enhancing the manipulation of fluids at the microscale.
