Interest in Lipid Nanoparticle generation has been growing over the past decade. Indeed, these Nanoliposomes have many advantages, such as the possibility of combining different therapeutic substances, protecting the active ingredients against alterations, controlling the diffusion of substances in the body, or even the possibility of complete sterilization by filtration.
What is a Nanoliposome?
Nanoliposomes or Lipid Nanoparticle are often composed of one or more lamellar layers. These layers include a phospholipid bilayer that contains a small volume of aqueous liquid. The diameter of nanoliposomes can vary from a few tens of nanometers to hundreds of micrometers depending on the method used to generate them.
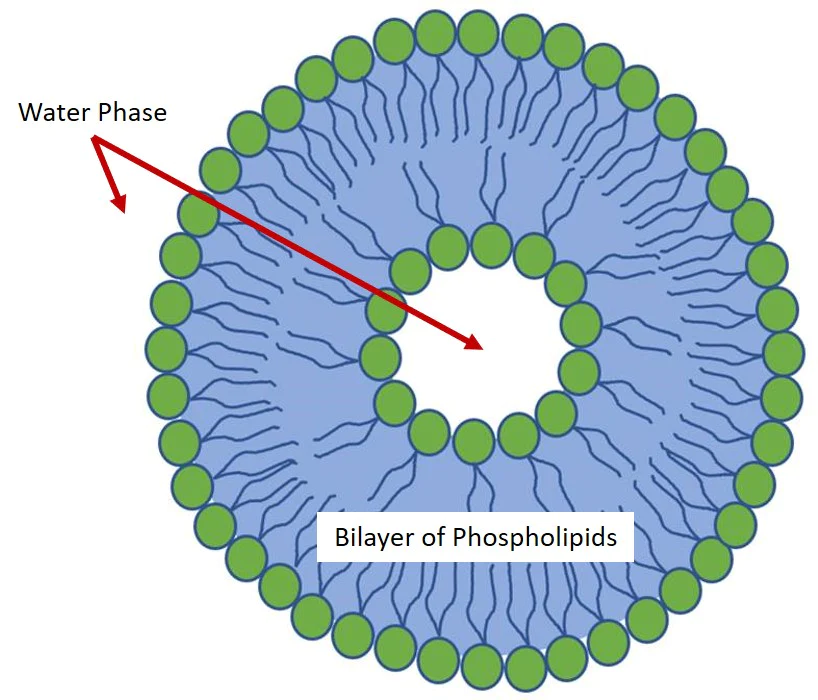
Phospholipids and lipids are the main components of liposomal membranes. They have the particularity of being soluble in various organic solvents and it is precisely for this property that most current methods use organic solvents to solubilize the lipids as the first step of the protocol.
The rise of microfluidics opens a new door for the preparation of lipid-based nanoparticles. Microfluidic methods have demonstrated their ability to control the mixing process of the organic and aqueous phases, but also the size and thus obtain a high monodispersity as well as the possibility to play on the design of the microfluidic chip to vary the composition and size of the obtained nanoliposomes.
Advantages of using microfluidics to generate Nanoliposomes
The oldest and traditional methods for the preparation of liposomes consist of the so-called macroscale or batch techniques based on the initial dehydration of lipid films followed by the swelling process and the final mechanical manipulation to create the water-dispersed bilayers. However, poor reproducibility, limited process control and the inefficient use of materials and reagents characterize these methods.
Microfluidics has been recently investigated as an efficient method for the preparation of liposomes in a controlled, reproducible and hight-throughput manner.
Microfluidics allows a reduction of the used volumes and the associated costs by handling fluids in geometrically constrained compartments typically characterized by micrometer length scales and low Reynolds numbers.
What microfluidic devices to use for Nanoliposome preparation?
Some chips may also have a specific treatment, which will influence the size or composition of the nanoliposomes. In this section, we will therefore look at the different geometries that exist and examples from the literature to assess the resulting applications.
Herringbone mixer for Lipid Nanoparticles production
Recently, micromixing through the Herringbone method has been investigated and shows great advantages among the other preparation methods in terms of liposome production efficiency.
For example, PEGylated liposomes were successfully prepared by using a Herringbone Mixer . This device is a chaotic advection mixer able to disrupt the laminar flow typical of microchannels, and reduce the time needed for the liposomes preparation process.
The specific pattern of the device made of herringbone grooves redistributes the fluids by creating a transverse flow into the microchannel. The vertices of the grooves are offset to approximately one third of the width of the channel and two counter-rotating vortices are created by the grooves. The subsequent set of herringbone groves with a mirrored orientation of the vortices allow the creation of a full mixing cycle as shown in the Fig.2.
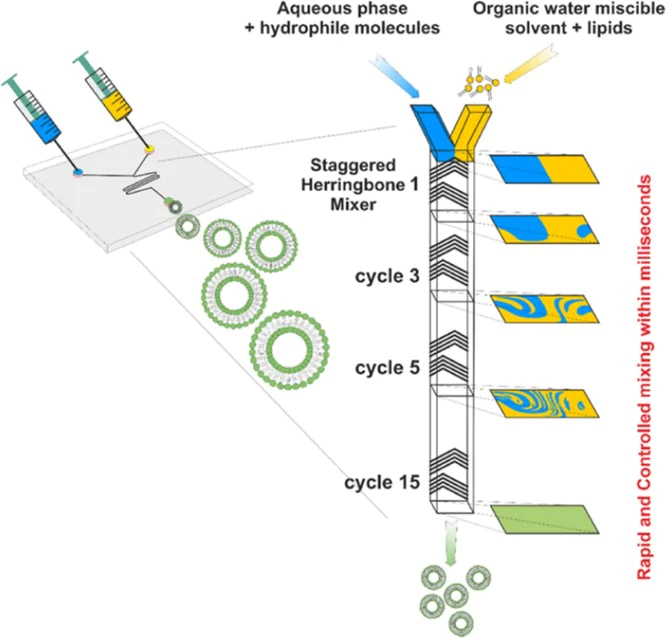
The alternation of these herringbone sets reorients the flow periodically and strongly improves the mixing. This microfluidic preparation method allows the controlled creation of liposomes of different sizes (∼100 nm) and good dispersity (< 0.2). By changing the flow rate parameters and the formulations in terms of aqueous buffer, phospholipid concentrations and composition it is possible to finely tune the production of liposomes with the desired features.
To illustrate this design, the team of Leug et al. (2015) proposes a general method for encapsulating macromolecules (SiRNA) in a lipid nanoparticle system. They were able to generate nanoparticles from 40 to 140 nm in diameter using a herringbone micromixer.
There are many techniques, and therefore many geometries, that allow you to generate nanoparticles. The information presented here will help you choose your microfluidic device according to your applications and the size of the nanoparticles you wish to generate.
Droplet generator for Lipid Nanoparticles production
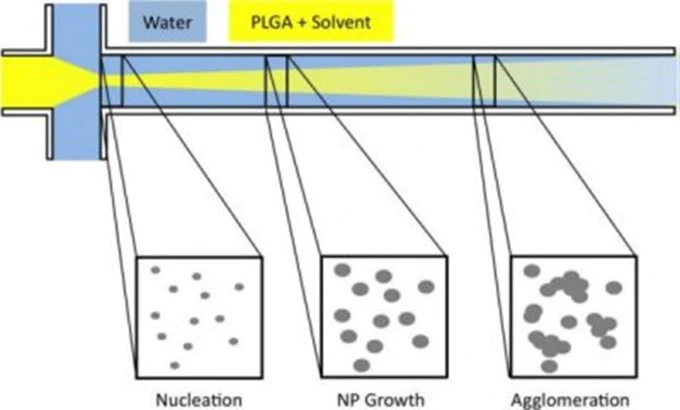
Typically, three flows merge in the same microfluidic channel as showed in Fig.3. This is the typical pattern of microfluidic devices suitable for the generation of droplets. As you can see a phospholipid alcoholic solution is injected in a central channel flanked by two lateral aqueous solutions.
The lipid containing stream is focused, and thanks to:
- The low Reynolds number.
- The diffusion dominated by the mass transfer.
The alcohol diffuses into the aqueous solution and its concentration in the central stream starts to decrease. As it reaches a critical concentration below the solubility of the phospholipids, these ones spontaneously organise into liposomes in a self-assembly manner.
The volumetric flow rate ratio between the lipid and water phase streams, together with the total flow rate, influence the size of the obtained liposomes and their adjustment allows a controlled preparation.
Finally, Karnik et al. showed in 2008 that it is possible to generate nanoparticles by flow focusing in a highly monodisperse manner. Indeed, the team is able to produce nanoparticles of 31 nm in diameter (± 1 nm). Here, the size varies according to the composition of the nanoparticles.
The Y-shaped microfluidic device for Lipid Nanoparticles production
This design allows two fluids (usually an aqueous solution containing surfactant and a lipid solution coupled to a solvent) to be brought into contact with each other and then allow nanoprecipitation to occur in the collection channel. Nanoprecipitation is the aggregation of nanoparticles in time and space.
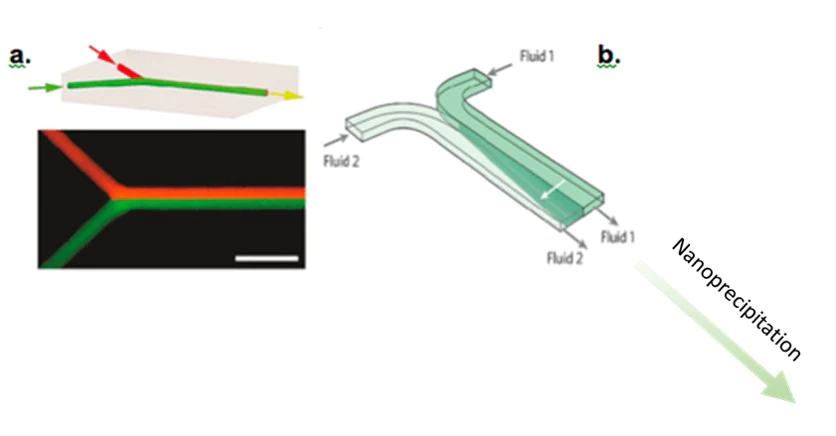
The Roces et al. team (2020) used a Y-shape micromixer to develop lipid nanoparticles. This device was used to generate nanoparticles of 100 to 200 nm in diameter. The size of these nanoparticles is considerably reduced after filtration, making it possible to obtain nanolipids between 50 and 80 nm with a polydispersity index of 0.2.
The 3D coaxial capillary device for Lipid Nanoparticles production
These devices offer the possibility of creating 3D coaxial flows, which are essential for rapid and uniform mass transfer. The co-current capillary microfluidic device consists of two capillaries. A tapered glass capillary was inserted into another larger cylindrical capillary with a coaxial alignment. The inner fluid (aqueous solution containing surfactant) flows inside the inner cylindrical capillary, while the outer fluid (a lipid solution coupled to a solvent) flows between the inner and outer cylindrical capillaries.
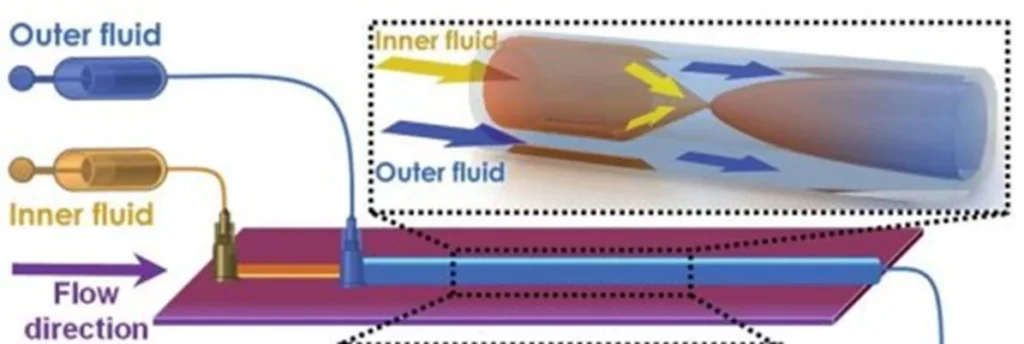
With this microfluidic device, Liu et al. published a paper in 2015 highlighting the generation of lipid nanoparticles between 70 and 280 nm in diameter with a polydispersity index close to 0.1. This size evolves as a function of the number of Renolds due to the presence of capillaries.
Final considerations
There are many techniques, and therefore many geometries, that allow you to generate nanoparticles. The information presented here will help you choose your microfluidic device according to your applications and the size of the nanoparticles you wish to generate.
Darwin Microfluidics is committed to this revolution. We provide innovative microfluidic devices for liposome preparation and our staff of experienced scientists and PhDs offer the support that researchers need to be successful in their activities.
🧠 What you have to remember
- Liposomes or lipid nanoparticles are enclosed vesicles widely used for the encapsulation of different kind of molecules such as DNA, proteins, drugs and/or other chemicals.
- Microfluidic methods offer an alternative way to prepare lipid nanoparticles with improved control over size and composition.
- Flow rate ratios between lipid and water phase streams, together with total flow rate, can be adjusted to obtain liposomes of desired size.
- By adjusting parameters such as the flow rate and formulation components, it is possible to achieve precise control over the size (<100 nm) and dispersity (< 0.2) of the liposomes.

