In microfluidics, it is essential that devices have a stable and well-characterized surface, especially when used for biomedical applications. We generally classify coatings between two groups: covalent and adsorptive. In this review, we will present the most relevant coatings used in microfluidics for different materials.
Which parameters influence the choice of coating?
The fluid that will pass through the channels of your device will be in close contact with the surface via the walls, and will therefore be very sensitive to the chemistry of the surface. Beyond chemistry, the material plays an extremely important role (find the application note on the different polymers used for the microfabrication of microfluidic chip here) because the way it reacts to the surface treatment is going to be a determining factor.
You then have to determine, of course depending on the application, the desired result. Indeed, the coating will be different if you want to make cells adhere to the surface, or just let a fluid flow to form drops at the outlet.
Finally, another parameter should be taken into account: surface tension. The change of scale due to miniaturization causes upheavals in the relative importance of the physical phenomena usually encountered on a macroscopic scale. Thus, in microfluidics, surface effects are important and cannot be neglected. The ratio of surface forces to volume forces will develop as the inverse of the characteristic length of the system. Its importance on the micrometer scale can lead to the appearance of particular phenomena such as Rayleigh-Plateau instability. In a nutshell, the instability of the R-plateau (Figure 1) states that a fluid cylinder deforms and fragments spontaneously to lower its surface energy. In a very general way, the film is unstable, it undulates at a certain wavelength. Then the undulations continue to grow and finally resolve into several droplets. The final state of instability is sometimes a succession of small drops called "satellites".
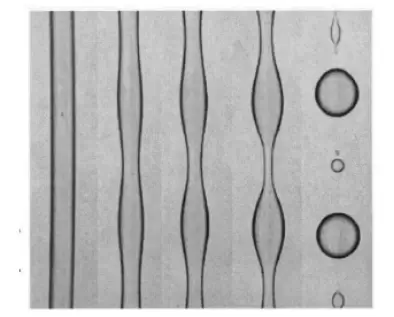
Having reviewed all the parameters to be taken into account, it is now time to detail the various types of coating.
The different types of coatings
Covalent coating strategies
Covalent coatings are characterised by a chemical bond formed between a functional group on the substrate surface, i.e. the walls of the microfluidic channels, and a functional group of the coating agent used. Covalent coatings are appreciated for their excellent stability. However, as they have to be adapted to the available surface functionality of the material used, this makes universal strategies more difficult to apply compared to other surface treatment methods.
Covalent coating strategies for PDMS
One of the covalent bonding strategies often used is to link alkoxysilane derivatives via surfaces bearing hydroxyl groups, thus obtaining siloxane bonds. This method was first used for glass and silica surfaces. Today, it is also used in microfluidic devices based on PDMS. The real advantage of this technique is the fact that the bond can be made via one to three alkoxy groups of the reacting silane. This then allows correct orientation and stable cross-linking at the surface. In addition, the functionality of the silane can be adjusted to achieve the desired chemical reactivity.
Covalent coating strategies for PMMA
Numerous activation and surface coupling procedures, allowing a wide range of functionalities, have been developed for PMMA consisting mainly of introducing amine functionalities on the PMMA surfaces (see figure 2). One is a coating of poly(ethyleneimine) (PEI), an amino polymer that is known to enhance antibody binding to the PMMA surface [Bai et al., 2006 Langmuir].
Another example of surface treatment of PMMA with amine functionalities is the activation of lithiated diamines to bind alkylcyanates to PMMA [Henry et al., 2000 Analytical Chemistry]. This method was used by Hashimoto et al. [2005 Analytical Chemistry, 2006 Biosensors & Bioelectronics] to improve the detection of single base DNA mutations.
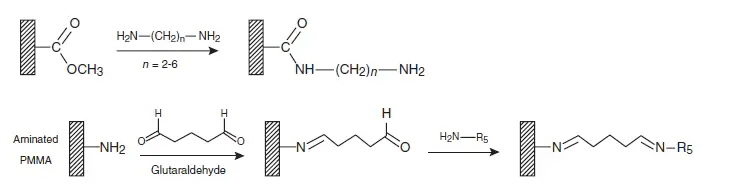
Covalent coating strategies Polycarbonate
One approach to covalent bonding on polycarbonate involves the creation of carboxylic groups on the surface. Thus, UV/ozone treatment can be used to induce COOH groups for the binding of amino groups via carbodiimide activation [Li et al., 2007 Analytical Chemistry].
The above-mentioned approach, which has given good results on PMMA, can also be used for polycarbonate [Hashimoto et al. 2005 Analytical Chemistry, 2006 Biosensors & Bioelectronics]. Surface activation with lithiated diamines can also be used with polycarbonate for DNA coupling on microfluidic surfaces to detect single base pair mutations and thus improve polymerase chain reaction (PCR) applications [Abdallah and Ros, 2013].
Covalent coating strategies Glass
Silicon dioxide or SiO2 surfaces are for sure the most studied surfaces for microfluidic applications. Indeed, their surface properties are suitable for a variety of applications such as microfluidics, capillary chromatography, or capillary electrophoresis. Glass, fused silica and quartz can all be derived using the same coupling chemistry.
The silanol groups on the surfaces of SiO2 react with the alkoxysilanes to form a stable covalent bond. Silanes similar to those used with PDMS is used for SiO 2 and create covalent reactions to bind to other entities depending on the silane head group used [Abdallah and Ros, 2013].
A slightly different application from silanization can be used to immobilize antibodies on glass beads in order to detect pathogens in a microchannel [Lee et al., 2006 Bull. Korean Chem. Soc.].
Adsorbtive coating strategies in microfluidics
Adsorption strategies are based on intramolecular interactions between the coating material and the substrate surface. They can be mediated by several means such as electrostatic interactions, van der Waals forces, and/or hydrophobic interactions. A major advantage of these coatings is their ease of use since the microchannels usually only have to be incubated with a solution containing the coating agent for a certain period of time. Adsorbtive coatings are widely used and perform as well as covalent coatings in terms of preventing biofouling and non-specific adsorption, as well as acting as binding molecules for better biomolecular fixation.
Protein coating in microfluidics
Protein coating is a very popular and widely used approach to coat the surfaces of microfluidic channels. For example, in a very classical way, coating with bovine serum albumin protein (BSA) has been used in molecular biology for several decades to block surface sites against non-specific adsorption.
Streptavidin is widely used as a binding molecule since it is highly affine in binding to biotin. The biotin-streptavidin bond is almost covalent and the small biotin molecule can be easily derivatized with other binding molecules or proteins [Abdallah and Ros, 2013]. Thus, this binding pair has found widespread applications in the physical and life sciences, and can also be used in adsorption strategies for microfluidic surface coatings. The use of a streptavidin derivative, NeutrAvidin, is a method for easily coating PDMS [Linder et al., 2001 Analytical Chemistry].
Adsorptive polymer coatings in microfluidics
The choice of the coating polymer is highly dependent on the microfluidic surface due to variations in surface interactions between materials. It is suitable for many applications and in addition, as glass, silica, and PDMS have similar surface chemistry, similar polymer-based coating strategies can be applied.
It has been proven that dynamic coating methods can be considered more stable than static immobilization. Indeed, with this method, the surface of the substrate is constantly renewed with coating material. In addition, several polymeric microfluidic materials have been subject to dynamic coating procedures.
For example, in the case of PMMA, it has been reported that the dynamic adsorbent coating of cellulose allows the long-term use (more than 100 passages) of a single microfluidic device for DNA electrophoresis [Abdallah and Ros, 2013].
Polyelectrolyte multilayers in microfluidics
Multilayer polyelectrolytes (PEM) are used for several applications. This method is defined by the transmission of interaction forces between the coating and the substrate surface through electrostatic interactions. This enables their stability. The advantage of this method is that it can offer a much longer service life compared to other physical adsorption strategies.
Other strategies of coating in microfluidics
The two categories listed above (covalent and adsorptive coating) include many of the surface treatments available for microfluidic devices. However, there are still other approaches that can be used to modify the surface properties of microfluidic devices, which we will detail briefly.
Exposure to reactive plasma gases can produce radicals that change the chemical composition of a surface. For example, it is common practice in microfabrication to use reactive oxygen species to modify PDMS and glass surfaces by UV or plasma treatment (by oxygen or ozone). In most cases, this type of treatment is followed by a covalent coating such as silanisation.
Another method can also be used: metal spraying. For example, the spraying and evaporation of gold on the surfaces of microchannels is a technique developed in microfluidics. The gold-coated surfaces serve as templates for self-assembled monolayers (SAM), to which groups of thiol heads can be applied [Abdallah and Ros, 2013].
Conclusion
Surface coatings are vital to the success of many microfluidic devices and for a variety of applications. The variety of materials and coating strategies for microfluidic materials is enormous, and we only have been able to introduce a few. However, with the parameters outlined in the first part of this review and the short application examples mentioned for each coating, you have all the keys to determine the ideal coating for your microfluidics platform.
For more information on possible applications depending on the coating, we invite you to consult the referenced articles.

