Surfactants are a diverse class of compounds that play a pivotal role in reducing the surface tension between two immiscible substances, such as oil and water. This unique property makes them indispensable for stabilizing droplets during emulsification. From pharmaceuticals to cosmetics and food production, surfactants enable precise control over droplet formation and stability, ensuring consistency and performance.
In this blog, we will explore what surfactants are, discuss their various types, and examine how their properties influence droplet generation and stabilization.
Physico-Chemical Definition of Surfactants
Surfactants, short for surface-active agents, are compounds that concentrate at the interface of two immiscible phases—such as oil and water—and reduce the surface or interfacial tension. This property is crucial for stabilizing emulsions, preventing phase separation, and enabling the formation of uniform droplets. The term "surfactant" reflects their ability to act at surfaces and interfaces, facilitating interactions between substances that typically resist mixing.
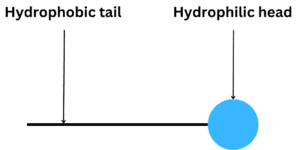
Surfactant molecules have a unique amphiphilic structure, meaning they contain two distinct parts:
- Hydrophobic (lipophilic) tail: This non-polar, water-repelling segment typically consists of one or more hydrocarbon chains. These chains can be linear, branched, or aromatic and are soluble in lipidic or oil phases.
- Hydrophilic head: This polar, water-attracting segment contains ionic or non-ionic groups, which readily interact with water molecules.
How Do Surfactants Work?
The dual nature of surfactants allows them to bridge the gap between polar (water) and non-polar (oil) phases. Surfactant molecules spontaneously adsorb at the interface of immiscible phases, with their hydrophobic tails immersed in the oil phase and hydrophilic heads in the water phase. This alignment reduces interfacial tension by minimizing energy at the interface and forms a stabilizing layer around each droplet.
This adsorbed layer acts as a barrier that resists compression and exhibits viscoelastic properties, enhancing droplet stability by preventing aggregation and coalescence.
The ability of surfactants to reduce interfacial tension and limit instabilities makes them indispensable in applications such as emulsification, droplet generation, and the stabilization of dispersed systems across a wide range of industries such as pharmaceuticals, cosmetics, and food production.
Different Types of Surfactants
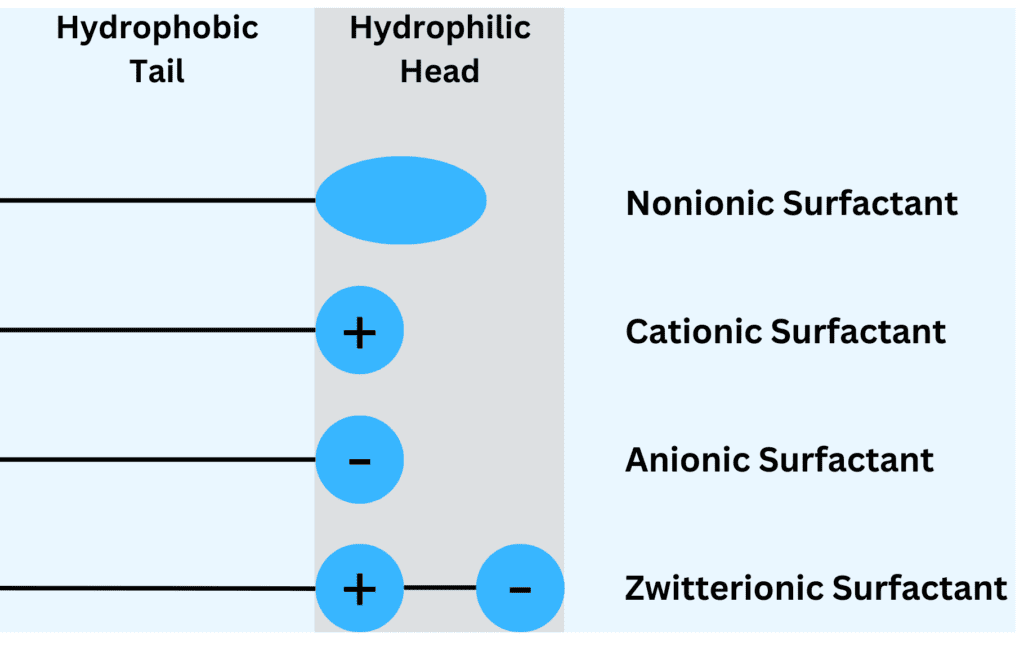
Surfactants are categorized into four primary types—anionic, cationic, nonionic, and zwitterionic—each distinguished by the nature of their hydrophilic head group. These variations in structure give each type unique properties, making them suited to specific applications in droplet generation and stabilization.
Anionic Surfactants
Anionic surfactants carry a negative charge on their hydrophilic head, making them particularly effective in environments where they can repel other charged particles. This negative charge generates electrostatic repulsion between droplets, effectively minimizing the risk of coalescence and ensuring a stable dispersion.
These surfactants are widely used in cleaning products, such as laundry detergents and shampoos. Their ability to form stable droplets makes them ideal for applications where detergent action and foam generation are essential.
Cationic Surfactants
Cationic surfactants have a positive charge on their hydrophilic head, making them particularly effective in applications requiring antimicrobial properties. Their positive charge allows them to interact with the negatively charged cell walls of bacteria, disrupting their structure and providing disinfection.
These surfactants are widely used in disinfectants, surface sanitizers, and cosmetic formulations. In droplet generation, they are especially valuable for products that benefit from antimicrobial action or conditioning effects, such as hair conditioners and personal care items designed for hygiene and care.
Nonionic Surfactants
Nonionic surfactants do not carry any charge on their hydrophilic head, making them suitable for a wide range of applications. This neutral nature allows them to interact with a broader range of substances without triggering electrostatic interactions, which can potentially destabilize droplets.
These surfactants are commonly employed as wetting agents and stabilizers in industries such as pharmaceuticals, food production, and cosmetics. Their ability to maintain droplet stability without altering the system’s pH or reacting with ions makes them an excellent choice for sensitive formulations.
Zwitterionic Surfactants
Zwitterionic surfactants possess both positive and negative charges on their hydrophilic head, enabling them to function as both anionic and cationic surfactants. This dual-charged structure imparts exceptional versatility and stability, allowing them to form highly stable droplets across a wide range of pH levels.
These surfactants are commonly used in personal care products and pharmaceutical applications. Their balanced charge makes them gentle and effective, and therefore ideal for sensitive formulations such as skincare products and biocompatible emulsions.
💡 Anionic and nonionic are the most widely used types of surfactants across industries due to their cost-effectiveness and versatility. In contrast, cationic and zwitterionic surfactants are typically reserved for specialized applications, as their production is more expensive.
Properties of Surfactants
The effectiveness of a surfactant in droplet generation depends on its intrinsic properties, which determine how well it stabilizes the droplets. Selecting the right surfactant for a specific application requires careful consideration of specific properties that directly influence efficiency, stability, and overall performance in droplet generation processes.
Hydrophilic-Lipophilic Balance (HLB)
The Hydrophilic-Lipophilic Balance (HLB) describes the balance between the hydrophilic and lipophilic parts of a surfactant molecule. It is critical in droplet generation as it determines the type and stability of the emulsion.
This balance is expressed as a numerical value on a scale (Davies scale), typically ranging from 1 to 20, which helps predict the behavior of the surfactant in different systems.
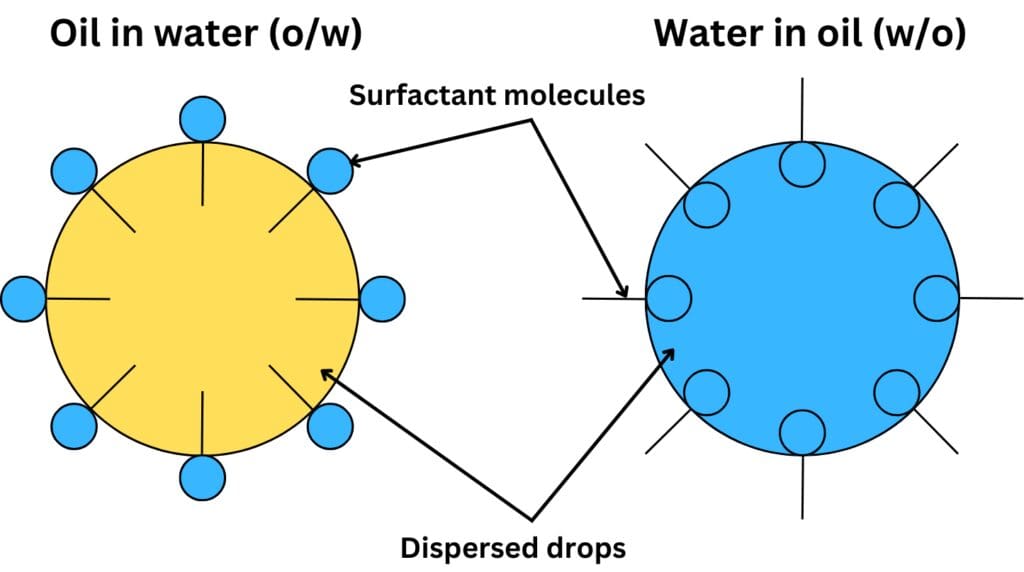
- Low HLB values indicate that the surfactant is more lipophilic and suited for stabilizing water-in-oil (W/O) emulsions.
- High HLB values suggest that the surfactant is more hydrophilic, making it ideal for oil-in-water (O/W) emulsions.
The following table outlines common surfactant applications based on their HLB range, highlighting how the balance between hydrophilic and lipophilic properties determines their functionality.
| HLB Values | Surfactant Applications |
|---|---|
| 1 to 3 | Anti-Foaming Agent |
| 3 to 6 | W/O (Water in Oil) Emulsifier |
| 7 to 9 | Wetting and Spreading Agent |
| 8 to 16 | O/W (Oil in Water) Emulsifier |
| 13 to 16 | Detergent |
| 15 to 18 | Solubilizer or Hydrotrope |
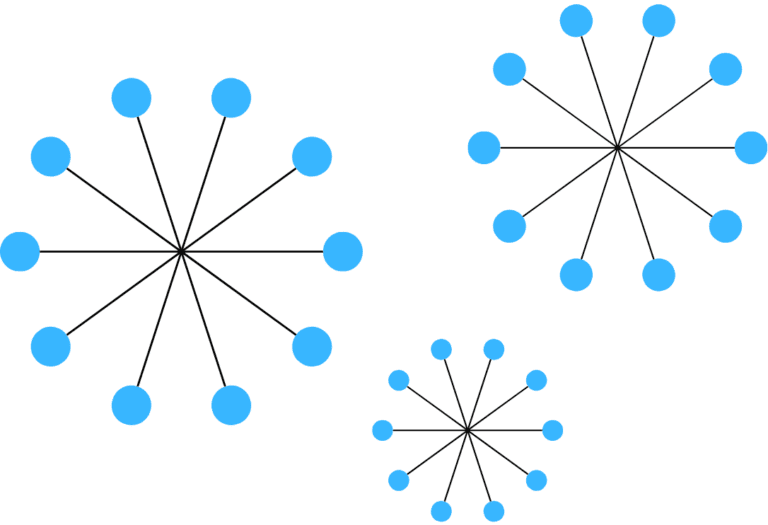
Critical Micelle Concentration (CMC)
The Critical Micelle Concentration (CMC) is a defining characteristic of surfactants, representing the concentration at which surfactant molecules transition from existing individually in a solution to forming micelles—clustered aggregates with their hydrophobic tails oriented inward and hydrophilic heads facing outward.
Below the CMC, surfactant molecules primarily work to reduce surface tension by adsorbing at interfaces. Once the CMC is exceeded, additional surfactant molecules contribute to micelle formation rather than further reducing surface tension.
Achieving the right balance around the CMC is crucial for optimizing droplet formation. Operating below the CMC may lead to insufficient stabilization of droplets, while exceeding the CMC can result in unnecessary surfactant usage without additional benefits. Moreover, excessive concentrations may cause issues like foaming, which can disrupt the droplet formation process, increase costs, and pose environmental challenges.
The CMC itself is not a static value; it is influenced by factors such as temperature, pressure, pH levels, and the presence of other substances like electrolytes. Understanding these variables helps in determining the optimal concentration for effective and efficient droplet generation.
Solubility
The solubility of a surfactant in both oil and water phases impacts droplet stabilization. Surfactants with high solubility in the continuous phase, tend to perform better at stabilizing droplets. This is because they easily migrate to the oil-water interface, where their amphiphilic nature allows them to form a stable film around droplets, preventing coalescence.
Selecting a surfactant with the appropriate solubility characteristics is essential. Surfactants that are well-soluble in the continuous phase tend to distribute more evenly throughout the system, leading to more uniform droplet sizes and enhanced stability. On the other hand, surfactants that are poorly soluble may fail to disperse adequately, leading to inconsistent droplet sizes or even phase separation.
Conclusion
Surfactants are essential for droplet generation, providing stability and versatility for a wide range of applications. By understanding their key properties, formulators can fine-tune emulsions for better stability and performance, ensuring optimal results in industries like pharmaceuticals and personal care.
Stay tuned for future content exploring surfactants, innovative droplet generation techniques, and their extensive applications across various industries😉. Until then, don’t pop your drop 🫧!
📧 If you have any questions or feedback, please feel free to contact us at contact@darwin-microfluidics.com.



