Liver plays a fundamental role in metabolic activities such as the regulation of glucose levels in the blood, production of plasma proteins, drug metabolism, production of bile [1][2] and presents a complex structure from a morphological and functional point of view [3].
Since its importance, it has been widely investigated in the past decades to understand the mechanisms of drugs metabolism and toxicity (DILI – Drug-Induced Liver Injury) as well as hepatic diseases such as hepatitis, cirrhosis, fibrosis and cancers [4][5][6].
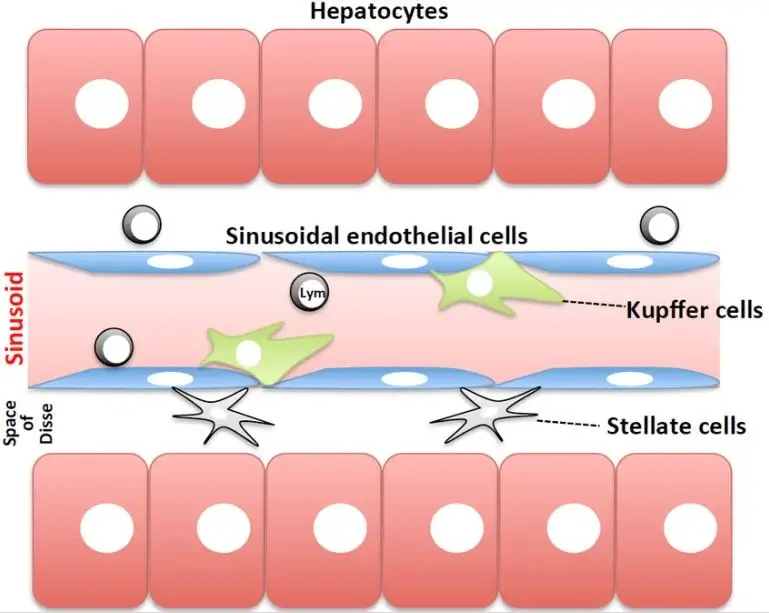
Short overview of the liver sinusoid structure
The hepatic portal vein (from the gut) and the hepatic artery take the blood to liver, where it is mixed in capillaries called sinusoids and collected in the hepatic central vein, where it is carried back to heart.
The liver is composed by repeating units, the lobules, with hexagonal shape, that consist of hepatocytes (HCs) arranged in cord structures around the sinusoids, separated by a perisinusoidal space, the space of Disse.
The hepatocytes are the parenchymal liver cells, mainly involved in proteins and bile synthesis and in the liver metabolism while the main non-parenchymal (NPC) cells are the liver sinusoidal endothelial cells (LSECs), the Kupffer cells (macrophages) and the stellate cells. Hepatocytes receive the nutrients from the sinusoidal cells through exchanges across the space of Disse [2] [7][8].
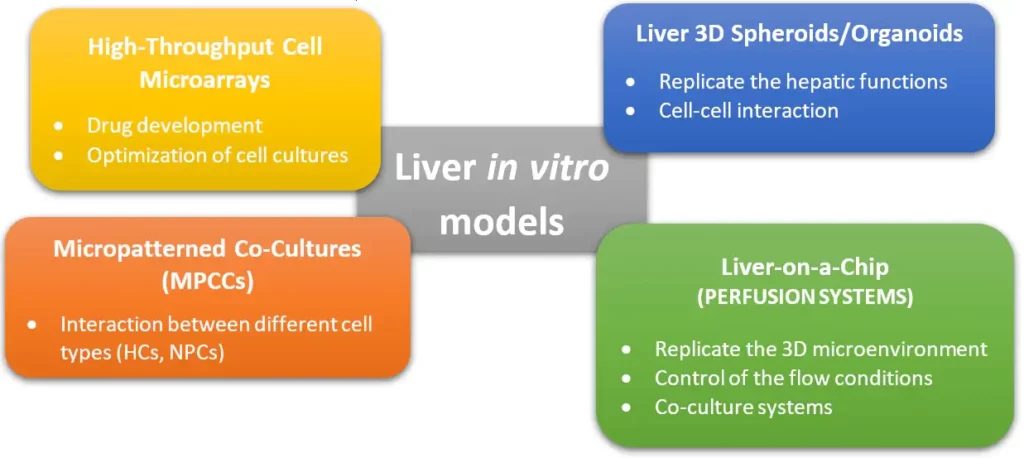
Engineered liver in-vitro models
A liver-on-a-chip model: The artifical liver sinusoid
We have decided to describe here the artificial liver sinusoid developed by Lee et al., who designed a microfluidic platform able to recreate a physiological microenvironment (mass transport conditions) for the seeding of primary hepatocytes [11].
Details on chip design
The microfluidic platform was composed of a channel for the flow of nutrients and drugs and a cell compartment for the seeding of tightly packed hepatocytes.
In fact, it has been demonstrated that high densities of primary cells allow the formation of cell-cell interactions with augmented viability and metabolic activity.
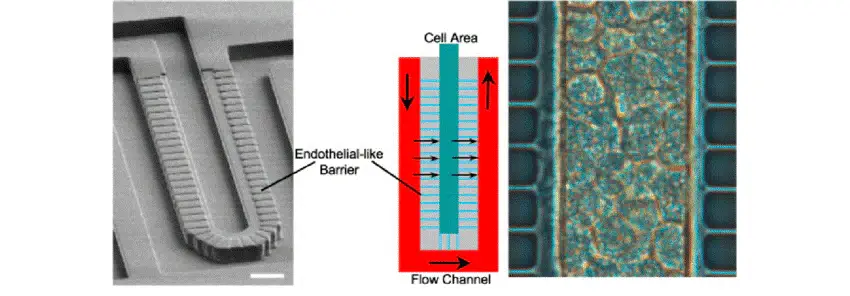
A permeable endothelial-like barrier with a sinusoidal shape composed of a set of parallel microchannels was included into the chip design in order to (i) prevent high shear stresses that can be dangerous for the HCs and (ii) pack the HCs in the cell area.
How to make a liver on a chip?
Chip microfabrication
SU-8 (a negative photoresist) was patterned through photolithography on a silicon wafer in two steps: a 1 µm thick SU-8 layer was spin-coated to create the channels of the endothelial-like barrier, then a 30 µm SU-8 was used to create the channels for the flow and the cells seeding.
Successively, poly-dimethyl siloxane (PDMS) chips were replicated from the SU-8 mold and bonded to a glass slide.
Designing the chip parameters to mimic the liver microcirculation
The chip was designed to ensure a nutrient volumetric flow of 100 pL/s (for about 250 hepatocytes loaded in the cell area), typical of liver microcirculation. To do so, the channels width, height and length were set at (50 x 30 x 1200) µm and (2 x 1 x 30) µm for the flow channel and the permeable barrier respectively.
Using a barrier channels cross-section of 2 x 1 µm, a low convective flow, so a high total fluidic resistance (1000 times higher than in the fluid channel) were ensured, thus enabling diffusive transport. In this way, HCs were exposed to low shear stresses and could receive nutrients through diffusion across the barrier.
Designing the chip parameters to control the loading of tightly packed hepatocytes
The design of the endothelial-like barrier also allowed the loading of a high density of HCs without damaging them or the barrier itself.
The narrow cross-section of the channels was verified to reduce the cells membrane deformation at the interface.
Thus, a positive flow directed the cells towards the bottom of the cell area without clogging, that resulted in a high cells packaging. Furthermore, an increasing resistance produced by the cells determined a decrease of the loading rate at the end of the seeding process.
Microfluidic chip for primary cells culture: Results and conclusion
Both rat and human primary hepatocytes were loaded within the cell area and the cell viability was assessed up to 7 days.
The microfluidic platform was continuously perfused with culture medium. Results shown that both the cell lines maintained their viability after 7 days with no need of collagen coating (necessary in static culture systems).
Moreover, the high density of cells ensured a high metabolic activity due to cell-cell contacts.
The review shows how microfluidic platforms can be engineered to closely mimic specific functions of a tissue or an organ. In this work, a microfluidic device composed of a flow channel, a cell area and a permeable endothelial-like barrier was designed to resemble the liver microcirculation conditions, improving the hepatocytes viability and the cell-cell interactions.
Review written by Alessandra Dellaquila.
References
[1] Y. B. Kang et al., “Liver sinusoid on a chip: Long‐term layered co‐culture of primary rat hepatocytes and endothelial cells in microfluidic platforms,” Biotechnol. Bioeng., vol. 112, no. 12, pp. 2571–2582, 2015.
[2] C. H. Beckwitt et al., “liver ‘organ on a chip,’” Exp. Cell Res., 2017.
[3] Y. Du et al., “Mimicking liver sinusoidal structures and functions using a 3D-configured microfluidic chip,” Lab Chip, vol. 17, no. 5, pp. 782–794, 2017.
[4] L. A. Vernetti et al., “A human liver microphysiology platform for investigating physiology, drug safety, and disease models,” Exp. Biol. Med., vol. 241, no. 1, pp. 101–114, 2016.
[5] V. Natarajan, E. N. Harris, and S. Kidambi, “SECs (sinusoidal endothelial cells), liver microenvironment, and fibrosis,” Biomed Res. Int., vol. 2017, 2017.
[6] S. S. Bale et al., “In vitro platforms for evaluating liver toxicity,” Exp. Biol. Med., vol. 239, no. 9, pp. 1180–1191, 2014.
[7] A. J. Strain and J. M. Neuberger, “A bioartificial liver–state of the art,” Science (80-. )., vol. 295, no. 5557, pp. 1005–1009, 2002.
[8] C. Moraes, G. Mehta, S. C. Lesher-Perez, and S. Takayama, Organs-on-a-Chip: A Focus on Compartmentalized Microdevices, vol. 40. 2011.
[9] H. Tsutsui and S. Nishiguchi, Importance of Kupffer Cells in the Development of Acute Liver Injuries in Mice, vol. 15. 2014.
[10] G. H. Underhill and S. R. Khetani, “Bioengineered Liver Models for Drug Testing and Cell Differentiation Studies,” Cell. Mol. Gastroenterol. Hepatol., vol. 5, no. 3, pp. 426–439, 2018.
[11] P. J. Lee, P. J. Hung, and L. P. Lee, “An artificial liver sinusoid with a microfluidic endothelial‐like barrier for primary hepatocyte culture,” Biotechnol. Bioeng., vol. 97, no. 5, pp. 1340–1346, 2007.