Definition of Hydrophobicity
Hydrophobicity is the physical property of a molecule or substance that causes it to repel water. It is a fascinating phenomenon with diverse applications, from designing water-repellent clothing to creating microfluidic devices.
Understanding Hydrophobicity
The term ‘hydrophobic’ comes from the Greek words ‘hydro’ (water) and ‘phobos’ (fear), describing substances that resist mixing with water.
Water molecules are polar, meaning they have distinct positive and negative ends, which allows them to form hydrogen bonds with other polar molecules, including other water molecules. In contrast, hydrophobic molecules are typically non-polar and lack such distinct ends, preventing them from forming hydrogen bonds with water. Consequently, hydrophobic substances are poorly soluble in water and tend to aggregate with other hydrophobic molecules to minimize their exposure to water, often leading to phase separation.
This behavior is crucial in the structure and function of biological membranes, protein folding, and the interaction of chemicals in aqueous environments, making hydrophobicity a fundamental concept in chemistry, biology, and microfluidics.
The Lotus Effect
A well-known example of natural hydrophobicity is the ‘lotus effect’. The leaves of the lotus plant are extremely hydrophobic, causing water to bead up and roll off, taking dirt and contaminants with it. This self-cleaning property has inspired the development of hydrophobic coatings and materials.
The lotus leaf achieves this high level of hydrophobicity through its surface structure. Tiny, waxy bumps on the leaf’s surface prevent water from spreading out, causing droplets to sit atop the bumps and roll off easily.
Measuring Hydrophobicity
Hydrophobicity is not a binary property; it exists on a spectrum. Some materials are more hydrophobic than others, and this can be quantified using the contact angle. The contact angle is the angle formed at the interface between a liquid/vapor and a solid surface. The higher the contact angle, the more hydrophobic the surface.
For instance, a perfectly hydrophilic (water-attracting) surface has a contact angle of 0 degrees, meaning water spreads out completely. In contrast, a highly hydrophobic surface may have a contact angle of 120 degrees or more, causing water to bead up and roll off.
Hydrophobicity in Microfluidics
One primary use of hydrophobicity in microfluidics is in the design of microchannels. By making channel walls hydrophobic, scientists can control the movement of water-based solutions, directing fluid flow, separating different fluids, or even creating droplets of one fluid within another.
Surface Modification Techniques
To create hydrophobic surfaces for microfluidics, various modification techniques are employed. One common method is chemical modification, where hydrophobic molecules are bonded to the surface. Techniques like silanization, where a silane molecule with a hydrophobic end is attached to the surface, are often used.
Physical modification is another approach, altering the surface structure to increase hydrophobicity. This involves creating micro or nanostructures on the surface, similar to the lotus leaf’s bumps, to raise the contact angle and enhance hydrophobicity.
Droplet Microfluidics
A particularly exciting application of hydrophobicity in microfluidics is droplet microfluidics. This involves creating, manipulating, and studying droplets within a microfluidic device. By combining hydrophobic and hydrophilic regions, scientists can form droplets of a water-based solution within an oil-based one, or vice versa.
These droplets serve as miniature reactors, enabling chemical or biological reactions on a microscale. This has numerous applications, from drug discovery to materials science, where precise control over droplet size and composition is essential.
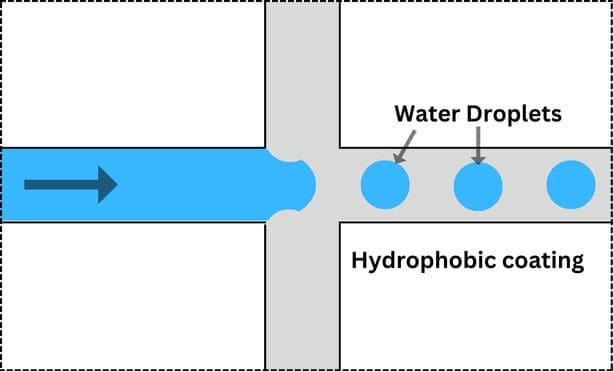
Practical Applications of Hydrophobicity in Microfluidics
Hydrophobicity has numerous practical applications, particularly in microfluidics. From medical diagnostics to environmental monitoring, the ability to manipulate fluids on a microscale is transforming many areas of science and technology.
Point-of-Care Testing
One key application of hydrophobic surfaces is in lab-on-a-chip devices, which integrate multiple laboratory functions onto a single chip, making testing faster, cheaper, and more efficient. These devices are particularly promising for point-of-care testing, where diagnostic tests are conducted at or near the patient care site. By using hydrophobic surfaces to control fluid flow, lab-on-a-chip devices can perform complex processes like DNA sequencing or immunoassays on a chip no larger than a credit card.
For instance, in a microfluidic device designed for blood glucose testing, a hydrophobic surface can separate blood cells from plasma, directing the plasma into a separate channel for analysis while discarding the cells. This ability to conduct quick, minimally equipped tests is especially beneficial in remote or resource-limited settings.
Environmental Monitoring
Microfluidic devices are also valuable in environmental monitoring, such as water quality testing or pollutant detection. These devices can react with specific substances, changing color or producing a signal when the substance is present. Hydrophobic surfaces help confine these reactions to specific areas, improving sensitivity and accuracy.
For instance, a microfluidic device detecting heavy metals in water might use hydrophobic surfaces to create water droplets within an oil-based solution. Each droplet acts as a miniature reactor, changing color if heavy metals are present, enabling rapid, on-site water quality testing.
Conclusion
Hydrophobicity is a fascinating property with wide-ranging applications, particularly in microfluidics. From controlling fluid flow in microchannels to creating miniature droplet reactors, manipulating hydrophobicity is revolutionizing fluid control on a microscale.
