Tiny but troublesome, air bubbles are a common headache in the field of microfluidics. These minuscule pockets of air can profoundly impact fluid dynamics within microscale channels, undermining device functionality and data accuracy. Understanding why these air bubbles form is indispensable for advancing strategies to mitigate their deleterious effects, making microfluidic devices work better and more consistently. One way of addressing this issue effectively is by using specialized equipments like our autoclavable bubble trap for microfluidics, designed to capture and remove these problematic bubbles.
In this blog post, we will explore the physics behind air bubble formation and examine the factors that contribute to their presence in microfluidic systems.
What Are the Physics of Air Bubble Formation in Microfluidics?
Understanding the physics behind bubble formation in microfluidic systems is essential for improving their performance and reliability. In this section, we will explore the physics that govern air bubble formation, with a focus on surface tension and nucleation theory.
Nucleation Theory and Bubble Initiation
Bubble formation in microfluidic systems starts with nucleation, where gas molecules coalesce to form a stable bubble. Nucleation can be homogeneous, occurring uniformly within the liquid, or heterogeneous, occurring on surfaces or impurities. Heterogeneous nucleation is more common in microfluidics due to constant fluid interaction with channel walls and microscopic contaminants.
In heterogeneous nucleation, gas molecules aggregate on surfaces or around impurities like rough surfaces, sharp corners, and particulate matter, which lower the energy barrier for bubble formation. The high surface-to-volume ratios in microfluidic environments accentuate the effects of these nucleation sites. As fluid flows through narrow channels, any microscopic irregularity, such as a minor wall imperfection or a small dust particle, can disrupt fluid flow and create localized areas of lower pressure, facilitating bubble formation.
Surface Tension and Bubble Formation
Surface tension, the cohesive force between liquid molecules at a surface, significantly impacts bubble formation in microfluidics by causing the liquid surface to contract and resist external forces. This force can trap air pockets, known as Harvey nuclei, particularly where fluid interfaces encounter irregularities or abrupt changes in channel geometry. These nuclei serve as seeds for bubble formation, and their presence is a direct consequence of the surface tension‘s interaction with the microchannel environment.
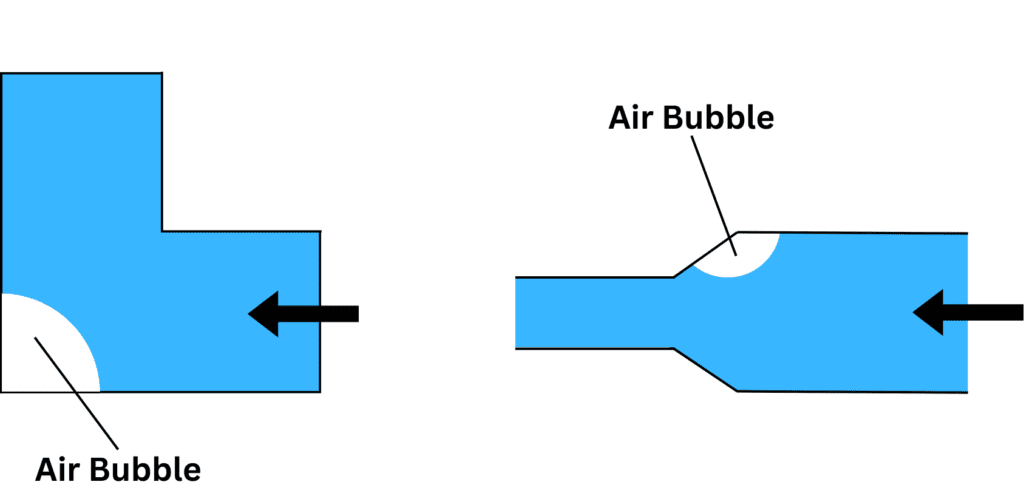
A key factor in air pocket formation and stabilization is the contact angle of the liquid, which measures how well the liquid wets the surface. A high contact angle indicates low wettability (liquid does not spread easily over the surface), allowing air pockets to stabilize and grow into bubbles, disrupting fluid flow.
The formation of these bubbles can be further influenced by several factors (detailed below) related to surface tension including channel geometry, surface roughness, fluid velocity and chemical composition.
What Are the Sources of Air Bubble Formation?
Several factors can contribute to the introduction and stabilization of air bubbles within microchannels, including chemical reactions, mechanical issues, and procedural errors. Below, we explore the primary sources of air bubble formation in microfluidic systems.
| Source of Air Bubble Formation | Description |
|---|---|
| Entrapped Air During Device Fabrication | Air can become trapped in microchannels during device sealing or bonding, leading to bubble formation when fluids are introduced. |
| Introduction of Bubbles During Fluid Loading | Improper priming of syringes or pumps can lead to the entrapment of air and can introduce air bubbles during fluid loading. |
| Leaking Connections and Faulty Fittings | Leaks in microfluidics systems due to faulty connections or fittings can introduce air bubbles. |
| Gas Solubility and Outgassing from Liquids | Fluids used in microfluidics often contain dissolved gases that can form bubbles when subjected to lower pressures or temperature changes, a phenomenon known as outgassing. |
| Chemical Reactions Producing Gas | Chemical reactions within microchannels can produce gases as by-products that can nucleate and form bubbles. |
| Material and Surface Properties | Hydrophobic materials and rough surfaces can trap air bubbles. Gas-permeable materials like PDMS can introduce bubbles, especially in long-term experiments. |
| Temperature and Pressure Variations | Changes in temperature and pressure alter gas solubility and can cause dissolved gases to come out of the solution and form bubbles. |
| Channel Geometry | Sharp corners, expansions, or contractions in the channel create conditions for bubble nucleation. Surface tension traps air pockets in these irregularities by causing the fluid to pull away. |
| Surface Roughness | Microscopic roughness on channel walls provides sites for air pockets to stabilize, allowing bubbles to grow over time. |
| Fluid Velocity | Variations in fluid velocity change pressure within microchannels and lower pressure areas can lead to bubble formation. |
| Chemical Composition | Surfactants or additives alter surface tension, either stabilizing or destabilizing bubble formation. |
Conclusion
In conclusion, air bubbles in microfluidic devices are a common but challenging issue, influenced by various factors including material properties, and operational conditions. Understanding the physics behind bubble formation and the sources of these bubbles is essential for optimizing microfluidic systems. Addressing these factors can improve the performance of microfluidic devices, paving the way for more accurate and efficient applications.
Stay tuned for future content exploring air bubble removal methods and their impact on microfluidic performance😉. Until then, no air bubble, no trouble 🫧!
📧 If you have any questions or feedback, please feel free to contact us at contact@darwin-microfluidics.com.

